
T
r
e
a
t
i
n
g
Depression
Ketabon – accessing ketamine’s
antidepressant potential
We develop a potential take-at-home treatment option for the 320 million patients suffering from Depression globally.
KET01 is an oral prolonged-release formulation of ketamine. It combines the robust and rapid efficacy of ketamine with superior tolerability, patient convenience, and accessibility compared to current treatment options. It therefore has the potential to be the first rapid-acting antidepressant as adjunctive therapy for Major Depressive Disorder (MDD) and/ or Treatment-Resistant Depression (TRD).
A growing burden
on patients and society
More and more people are suffering from neuropsychiatric disorders, globally. Especially burdensome for patients and society is Depression, having immense societal and economic costs which currently are not addressed adequately.


Mental Disorders

Treating
Depression
Ketabon aims to unlock ketamine’s full antidepressant potential while at the same time substantially limiting its side effects. We are developing an oral prolonged release formulation of ketamine (KET01) that has the potential to overcome the drawbacks of other ketamine formulations.
Arguably the greatest breakthrough
in the field of depression in
over 60 years
Ketamine is known to have significant antidepressant potential. Its rapid antidepressant effects in TRD-patients especially through intravenous infusions are well studied. Patients describe improvement of depressive symptoms within a matter of hours, which last up to one week. While efficacious, currently applied ketamine treatments including intranasal esketamine come with several limitations.
Change the future of
Depression Treatment with us
We are always open to new partners and fellow pioneers to share our vision of transforming the way we treat Depression. Join us today or contact us for more information.
Katharina Schwabe

About
Ketabon
Ketabon is a joint venture between HMNC Brain Health and Develco Pharma. The company is developing an oral prolonged-release formulation of ketamine for Major Depressive Disorder and Treatment-Resistant Depression. This innovative formulation aims to combine the rapid onset of efficacy of ketamine with enhanced tolerability and convenience, addressing key limitations of existing treatments. By minimizing dissociative and cardiovascular side effects through its unique pharmacokinetic profile, Ketabon offers a safer alternative to intravenous and intranasal ketamine therapies. Unlike current options that require strict medical supervision, this formulation has demonstrated potential for safe, unsupervised administration in clinical trials. With an estimated 320 million people suffering from Depression globally, and 94 million thereof being treatment resistant, the demand for rapid and effective solutions is critical. Ketabon’s oral prolonged-release ketamine has the potential to be used as adjunctive treatment during the first weeks of treatment with a conventional antidepressant. It also holds promise for synergistic efficacy when combined with conventional antidepressants, potentially offering a transformative solution for long-term treatment. Clinical development of oral prolonged-release ketamine could potentially be expanded into other indications.

Dr. Markus Zimmer
Co-CEO

Dr. Maximilian Döbler
Co-CEO

Dr. Hans Eriksson
CMO
Potential rapid-acting
treatment option
The clinical data in TRD to date strongly suggest KET01 as a potential rapid-acting treatment option for Depression. The early read-out from a Proof-of-Concept investigator-initiated clinical trial in 27 TRD patients reaffirmed KET01’s efficacy and tolerability potential.
mean MADRS score,
Day 15
Phase 2 trial
Based on the promising results from the Proof-of-Concept trial, we initiated a multicenter, double-blind, randomized, placebo-controlled Phase 2 trial in Germany, the Czech Republic and Poland. The study investigated the efficacy, safety, and tolerability of add-on treatment with ketamine hydrochloride prolonged-release tablets (KET01) in patients suffering from Treatment-Resistant Depression.
Head-to-head Trial of Prolonged-Release Oral Ketamine Formulation,
KET01, and Intranasal SPRAVATO®
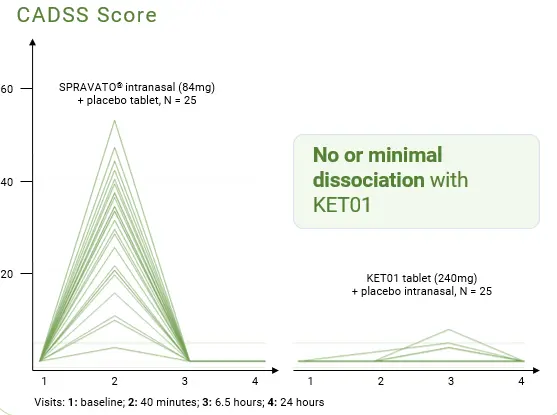
The Phase 1 trial compared 240 mg KET01 tablets to SPRAVATO® (intranasal esketamine) in 25 healthy male participants. Participants receiving KET01 experienced no or minimal dissociation—mean maximum CADSS score at post administration assessments until 24 hours after dosing was 29.6 for SPRAVATO® and 0.7 for KET01 (p < 0.001).
Strong Potential in the Early Treatment of MDD,
Addressing a Serious Unmet Medical Need
The findings inspired the extension of Ketabon’s strategy beyond Treatment-Resistant Depression to the broader Major Depressive Disorder indication.
KET01’s proprietary formulation effectively separates ketamine’s rapid antidepressant properties from its undesirable dissociative and cardiovascular side effects. Any patient beginning treatment with antidepressants or transitioning to a new antidepressant may therefore benefit from KET01 as a rapid-acting adjunctive treatment for MDD.
Entering Pivotal Phase 3 Clinical Trials
and targeting market entry by 2028
Building on promising clinical data, KET01 is now progressing to pivotal Phase 3 trials to evaluate its efficacy, safety, and tolerability as a rapid-acting adjunctive treatment for Major Depressive Disorder (MDD).
The study is expected to confirm the results of the previous trials, showing early-onset of efficacy, placebo-level dissociative side effects, and excellent tolerability, as well as potentially reveal synergistic efficacy with conventional antidepressants. Enabling safe, unsupervised use, KET01 holds first-in-class potential as a rapid-acting adjunctive therapy in MDD. Market entry is anticipated by 2028.
